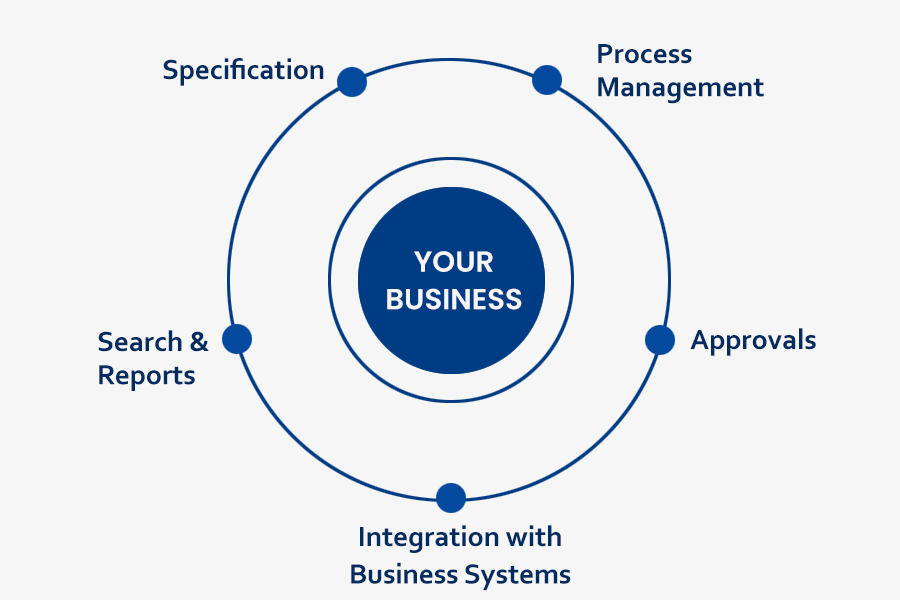
The benefits of a robust pharmaceutical artwork management system

Investing in a robust and streamlined process for artwork management can result in significant cost savings in a stringently driven regulatory environment. Not only do pharmaceutical companies have to incur effort and costs towards compliance, but they also need to reckon with the profound impact of non-compliance on cost, reputation, and patient safety.
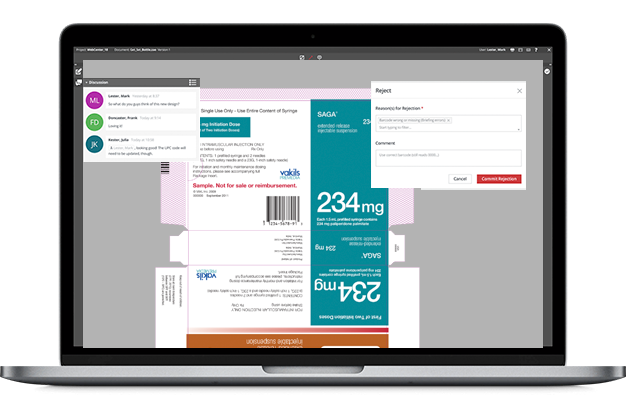

When it comes to artwork packaging and management, it looks deceptively like an innocuous, back office function. Nothing could be further away from the truth.
The primary objective of pharmaceutical artwork management is to experience zero product recall. Faster turn-around time, greater transparency and a comprehensive system that enables multi-language, multi-cultural and multi-regulatory environments are other key objectives.
This is where a comprehensive AMS steps in. It enables pharmaceutical companies to prevent errors. Specialist partners can leverage their domain and process expertise to help pharmaceutical organisations with innovative, scalable and cost-effective solutions and services.
Such a process includes a well-integrated workflow comprising:
- Pre-submission check – for template adherence, consistency and content accuracy check
- Post-submission check – for artwork design and approval, and further content verification
- Quality analysis on content and format – using relevant and comprehensive checklists
Digital packaging management systems can ensure control, quality, compliance and visibility to packaging and labeling processes. They can bring automated templates, structured content authoring (SCA), robotic process automation (RPA), and e-labeling. This, in turn, increases productivity, improves compliance, and brings down costs substantially.